manufacturing logbook|eLogbook (Electronic Logbook) : Bacolod Managing Your Manufacturing with Logbook. There’s a wide range of compliance areas that directly (or indirectly) affects manufacturers. These can include: . Cryptocurrency exchanges that uses maker / taker fee model often charges little to no fees for the maker orders (limit orders) and a slight higher fee for taker orders (market orders). It is very important that .
PH0 · eLogbook (Electronic Logbook)
PH1 · Why Automating Logbooks Should be the First Step to Manufacturing
PH2 · Why Automating Logbooks Should be the First Step to
PH3 · WHAT IS A MANUFACTURING LOGBOOK ? DETAILED VERSION
PH4 · WHAT IS A MANUFACTURING LOGBOOK
PH5 · Understanding the GMP Logbook Requirements
PH6 · ShiftNote
PH7 · Manufacturing eLogbooks Better Than Paper
PH8 · Manufacturing E Logbook Benefits
PH9 · Managing Your Manufacturing with Logbook Software
PH10 · Electronic Logbook (eLogBook)
Welcome to Murlok.io's PvE guide for Elemental Shamans in Mythic+ Dungeons. This guide covers the best stats, races, talents, gear, embellishments, enchantments, and gems for Patch 11.0, based on data from the top 50 Elemental Shamans in The War Within Pre-Patch across the US, EU, KR, and TW regions.
manufacturing logbook*******A manufacturing production log, is an excellent way of recording the ‘stage by stage process of manufacturing a product. In its simplest form, it is a series of photographs accompanied by notes. A complex form, will be .
Managing Your Manufacturing with Logbook. There’s a wide range of compliance areas that directly (or indirectly) affects manufacturers. These can include: .manufacturing logbook eLogbook (Electronic Logbook) Logbook tasks are cumbersome and time-consuming. Any number of issues can occur with a paper-based manufacturing .
Electronic Logbook Software. Maximize efficiency and streamline your manufacturing plant's operations with our state-of-the-art Logbook Software. Experience the power of .Electronic Log Book Applications. eLogBook can be used to: Record and manage both events and instructions. With its simpified data-entry process requiring minimal keyboard .
Rather than hunting for the right logbook for a piece of machinery and then wondering if it’s the right one, manufacturing logbook software can integrate with key workflows, production records, and . Understanding the GMP Logbook Requirements. 5 April 2024. In regulated industries such as pharmaceuticals, food and beverages, cosmetics, medical devices, .Logbook digitalization helps users record task details in real-time, track issuance and retrieval of logbooks, reduce transcriptional and misleading entries, and ensure data .Caliber’s eLogbook (Electronic Logbook) easily automates manufacturing logbooks. Entries made in the eLog are real-time and ensures review, verification, and approvals .While manufacturing a batch of products, a usual Batch Manufacturing Record (BMR) varies from 200 to 400 pages. Of this, logbooks comprise about 20-40% of the volume. .
Untuk kegunaan Pegawai Farmasi Provisional (PRP) yang menjalani latihan provisional di sektor awam (ambilan 1 Mei 2023 dan ke atas) Mengandungi sembilan (9) modul latihan iaitu: Ward Pharmacy Practice Out-patient Pharmacy Services In-patient Pharmacy Services Drugs and Poisons Information Services Manufacturing and .
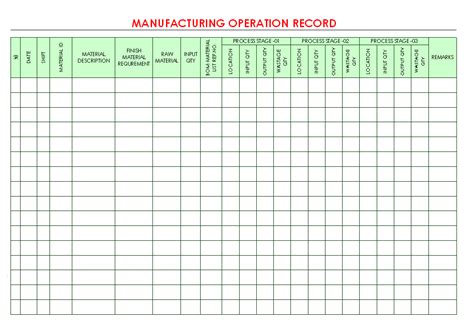
Manufacturing logbook software can offer flexible, no-code form builders that make it easy to make changes when necessary. Keep Data at Your Fingertips. It can be very (very) difficult to track down a .The logbook must be labelled on the front cover with the following information: • Team • Line name • Title of the logbook (i.e. Register, Communications) • Year • Date issued All pages in the logbook are numbered sequentially. No pages are to be removed from logbooks. Correction to a logbook should follow the requirements of GMP and. SOPBenefit from a SingleSource of Truth. Automatically populate shift logs based on data captured through permits, operational observations and work planned for the next shift. Our software helps keep your people and assets safe by ensuring that everyone knows what’s happening, where and when it’s happening, and what’s driving the risk .
Logbook is a perfect complement to your warehouse operations. We designed the software to make collaboration easy, clear and mobile. Assign templates by employee, push out reminders to specific members of the team and turnover notes between shifts, and even create equipment parameters. Communication is simplified, increasing efficiency and .Good manufacturing practice. Good manufacturing practice (GMP) describes the minimum standard that a medicines manufacturer must meet in their production processes. The European Medicines Agency (EMA) coordinates inspections to verify compliance with these standards and plays a key role in harmonising GMP activities at European Union .
The Vimachem Digital Forms and Logbooks module is a cloud-based forms & logbooks design and bookkeeping solution that digitizes within minutes any paper-based logbook and form found in pharma and biotech sites while complying with Current Good Manufacturing Practice (CGMP). Digital pharma logbook enables real-time data .
The eLogbook is completely compatible with Windows OS based and tablets. The production team members can use eLog compatible tablets and other appliances on the spot to make entries and reviews. Caliber’s eLogbook (Electronic logbook) easily automates manufacturing logbooks. It ensures review, verification, and approvals on .
An equipment maintenance log is used for record keeping maintenance activities done with equipment. Download and customize this maintenance record template to keep a record of maintenance works. This equipment maintenance log template includes the following fields: Date & time of maintenance activity. Maintenance .
For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 211.182 Equipment cleaning and use log. A written record of major equipment cleaning, maintenance (except routine maintenance such as lubrication and adjustments), and use shall be included in individual equipment logs that .Versify Digital Logbook Rapid offers a cost-effective electronic logbook, configurable forms and workflow capability for small-to-medium sized energy, utilities, oil and gas, water treatment, industrial and manufacturing companies. An exceptionally flexible and easy-to-use fully hosted solution.eLogbook (Electronic Logbook)2.4 This record book will be used for the purpose of appraisals by the Principal Preceptors and Master Preceptor, and will be submitted to the Pharmacy Board for the registration of the PRP as a fully registered pharmacist. 2.5 There are 6 main modules of training for the provisionally registered pharmacist [PRP] in the pharmaceutical industry;ProsperForms — receive reports from your team members on autopilot. 100+ forms available: reports, logbooks, requests, etc. or build your own. View and manage data on Timeline and Dashboard screens, generate consolidated PDF reports.manufacturing logbookShift Handover Made Better. With technology at our fingertips, there is a better way to capture and pass on information from shift to shift. Digital logging systems like Logbook, empower operators with a way to easily collect and communicate data throughout their teams in real time. Templates can be customized for each operations specific use case.The basic rules in any good manufacturing practice (GMP) regulations specify that the pharmaceutical manufacturer must maintain proper documentation and records. . Typically, logbooks are used for documenting the operation, maintenance, and calibration of a piece of equipment. Logbooks are also used to record critical activities, e.g .Logbook. Home; SAP S/4HANA; Product Lifecycle Management (PLM) Maintenance, Repair, and Overhaul (MRO) Maintenance and Service Processing; Logbook; Product Lifecycle Management (PLM) 2023 Latest. Available Versions: 2023 Latest ; 2023 Latest ; 2022 Latest ; 2022 FPS02 (May 2023) 2022 FPS01 (Feb 2023)

3. Assessment criteria. The assessment criteria should include the following: (i) supporting schedules and calculations for the preparation of financial reports. 4. Evidence. Copy of the supporting schedules and the drafted financial reports, the assessments must focus on the accuracy of the supporting schedules.Superior Data Management with AmpleLogic eLOGS. AmpleLogic eLogbook revolutionizes data management in regulated industries such as Life Sciences, Food & Beverages, Cosmetics, Gene Therapy, Medical Devices, and more. Our web-based software replaces manual logbooks, guaranteeing compliance, efficiency, and .
Al centro degli eventi della serie tv turca "Yargi" c'erano due avvocati piuttosto affermati. Ogni volta iniziano la loro nuova attività con grande entusiasmo e si sforzano di portarla a termine vittoriosamente. Gli eroi della serie tv turca, che si può guardare in italiano, hanno un
manufacturing logbook|eLogbook (Electronic Logbook)